
On behalf of the United States of America. 94.20 g/mol Computed by PubChem 2.1 (PubChem release 2021.05.07) Dates Create: Modify: Description Dimethyl disulfide appears as a colorless oily liquid with a garlic-like odor. Shall not be liable for any damage that may result fromįor NIST Standard Reference Data products. However, NIST makes no warranties to that effect, and NIST Uses its best efforts to deliver a high quality copy of theĭatabase and to verify that the data contained therein haveīeen selected on the basis of sound scientific judgment. The National Institute of Standards and Technology (NIST)

When re - leased across the air-sea interface, it plays several important roles in the atmosphere, including affecting the. A reevaluation of the upper proton affinity range, Dimethylsulfide (DMS) is a gas produced biologically in the oceans. Proton affinity ladders from variable-temperature equilibrium measurements. Peschke, M.,Ī Definitive Investigation of the Gas-Phase Two-Center Three-electron Bond in, +, and +: Therory and Experiment, Mutual Effects of Weak and Strong Ligands in Mixed Clusters,ĭeng, Y. The Ionic Hydrogen Bond and Ion Solvation. Gas phase chemistry of alpha-thio carbanions,Ĭan. Photoelectron Spectroscopy of Sulfur Ions, Go To: Top, Reaction thermochemistry data, Notes
DIMETHYL SULFIDE CHARGE FREE
C 2H 6S ) Free energy of reaction Δ rG° (kJ/mol)īy formula: HI + C 2H 5IS = C 2H 6S + I 2 Quantity.Gas phase switching reaction(Li+)H2O, from graph Dzidic and Kebarle, 1970 extrapolated M Liquid phase Reanalyzed by Cox and Pilcher, 1970, Original value = -278.3 ± 0.8 kJ/mol At 291°K ALS Gas phase Δ rH?, inconsistent with other protonated sulfur dimers Mīy formula: C 2H 6S + + C 2H 6S = ( C 2H 6S + īond type: Charge transfer bond (positive ion)īy formula: C 4H 9 + + C 2H 6S = ( C 4H 9 + īy formula: 2 C 2H 6S + O 2 = 2 C 2H 6OS Quantity Reaction search pages in place of the enumerated reactionīy formula: C 2H 5S - + H + = C 2H 6S Quantityīy formula: C 2H 7S + + C 2H 6S = ( C 2H 7S + Secretary of Commerce on behalf of the U.S.A. Your institution may already be a subscriber.įollow the links above to find out more about the dataīy the U.S. With the development of data collections included in bond in dimethyl sulfoxide is highly polar, with a positive charge on. The purpose of the fee is to recover costs associated Explain why dimethyl sulfoxide (bp 189C) has a much higher boiling point than. NIST subscription sites provide data under theĭata Program, but require an annual fee to access.
DIMETHYL SULFIDE CHARGE PROFESSIONAL
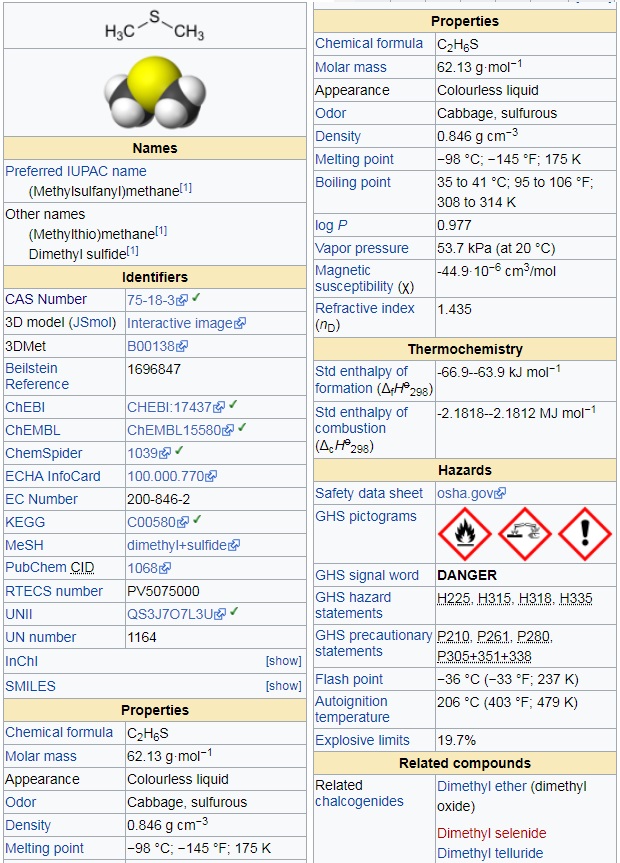
Use this link for bookmarking this species This structure is also available as a 2d Mol file IUPAC Standard InChIKey: QMMFVYPAHWMCMS-UHFFFAOYSA-N Copy.Given the physical data of two bond angles practically equal (what is $1^\circ$ anyway?), I conclude the question is based on a false premise and can be challenged. We can immediately discard $\ce) = 98^\circ 52'$ - again neither of which being consistent with non-hybridisation.


 0 kommentar(er)
0 kommentar(er)
